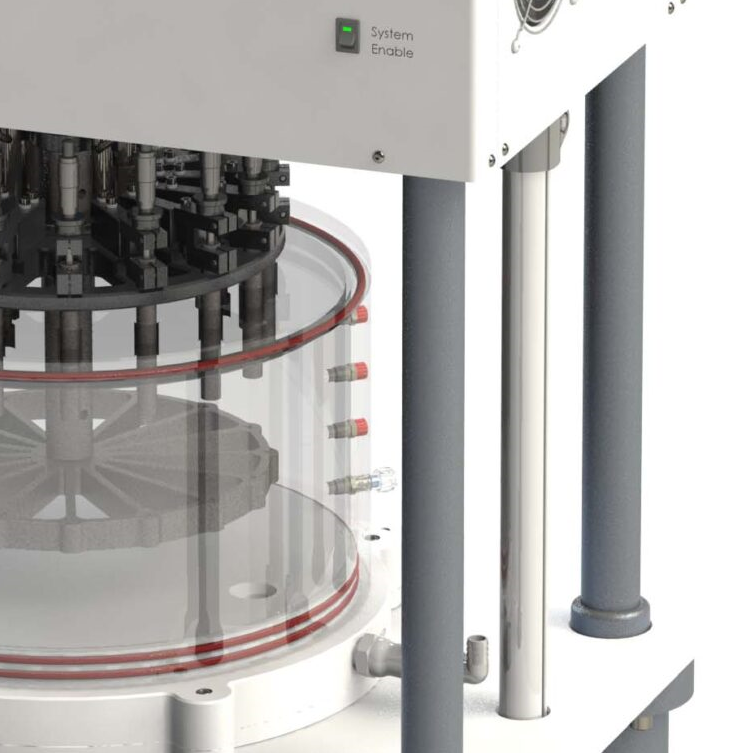
BDC Laboratories’ testing equipment is conceived, designed, and manufactured from an academic research foundation, and then coupled to industry R&D and regulatory expectations. This paradigm shift in medical device test equipment has facilitated a generation of test systems that allows for ultimate adaptability, comprehensive test sample monitoring and data-rich test environments.
Connect with BDC
BDC Laboratories most significant strength is their knowledge of medical device testing. Since they use the testing equipment and mock vessels they market, they understand the capabilities and limitations of their product. BDC Laboratories cares about their customers and we order with confidence knowing they will meet our most important deadlines. BDC Laboratories understands the current regulatory environment of the medical device industry and actively participates in ISO and ASTM standards development. This means the testing they provide is more likely to meet dynamic regulatory requirements.
Cook / Med Institute, Inc
Read More