Complex, Critical Test Services for Medical Devices
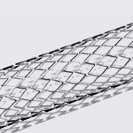
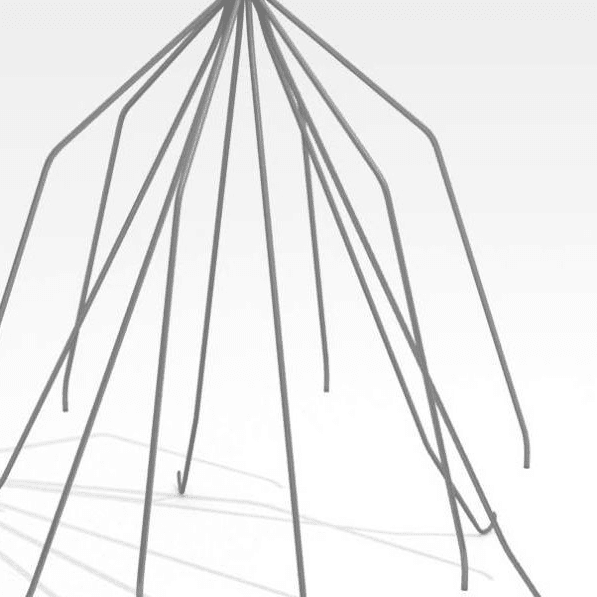
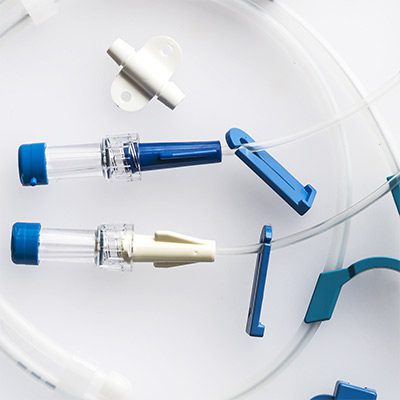
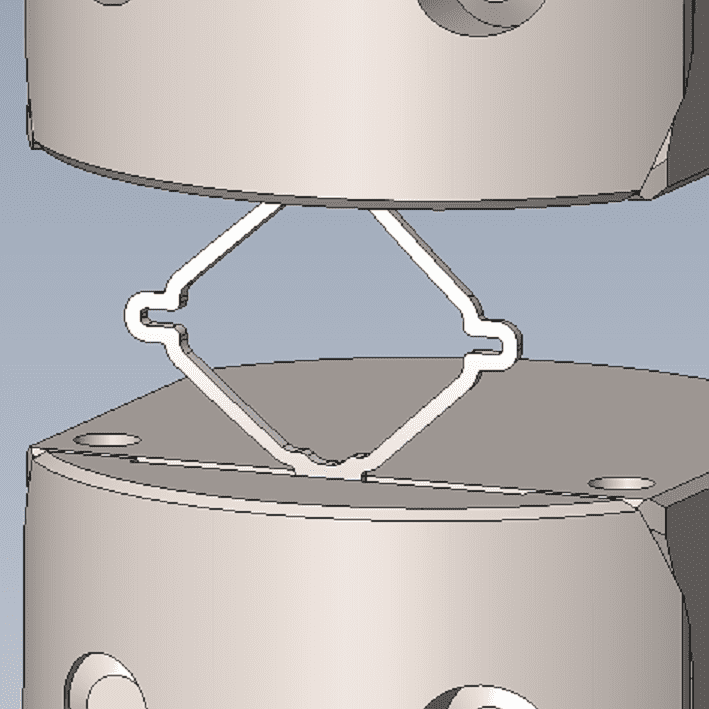
While some companies chose to test their devices in house, many look to the experts at BDC Laboratories for testing services. BDC Laboratories scope of Accreditation to ISO/IEC 17025:2017 includes Mechanical Testing of Endovascular Devices, Cardiac Valve Prostheses, Vascular Prostheses, and general Medical Devices. By using BDC testing services, organizations gain access to compliant systems and the flexibility to move through the regulatory process quickly.
The BDC Labs team has over 40 years of experience in medical device research, development, and testing. Our focus is on functional, engineering based testing of medical devices including Class 2 and Class 3 implants. The BDC team actively participates in The American Association of Medical Instrumentation (AAMI), the International Standards Organization (ISO) and ASTM Endovascular Device and Stent Standards Committees. As participants on these committees, BDC personnel have a unique understanding of developing medical device testing and verification plans, along with protocols to support FDA and CE Mark submissions on many types of medical devices. BDC has a fully established quality system based on Good Laboratory Practices (GLP), as well as accredited to ISO/IEC 17025:2017.
Connect with BDC
Benefits Partnering with BDC
- Team active in international standards development
- Extensive experience in FDA and worldwide regulatory submissions
- Rapid attention to all requests
- Established and audited ISO 17025 & GLP testing services
- Customer service as a core value
- Extensive product development experience
- Complete confidentiality
General Capabilities
BDC is a great, reliable contract laboratory. They exhibited great technical proficiency and tailored the test protocols to our specific samples. The customer service was exceptional as well. They worked with us to accommodate our timelines despite receiving a portion of the test samples last minute.
Medtronic (Cardiac Division), Inc.
Read More