About BDC Laboratories
Who we are and how we got here
What We Do
Medical device development is complex, and the testing process is similarly intricate. Keeping strict quality standards in mind, BDC Laboratories partners with R&D engineering teams to establish a flexible path to reliable, timely testing for medical implants and their delivery systems. Supporting clients through the entire development cycle, we design and develop precise silicone vessels for simulation and training when it’s time to go to market.
Who We Are
We’re a team of technical experts that’s energized by the goal of helping our partners develop medical devices that improve healthcare outcomes for patients all over the world. Known for our extensive experience in cardiovascular and endovascular device evaluation, we work with engineering teams to test medical device designs through testing services and fully delivered test systems. We are also active participants in the AAMI, ISO, and ASTM International’s Endovascular Device and Stent Standards committees, providing technical expertise to support the ongoing improvement of medical device quality and effectiveness.
Our Mission
The mission of BDC Laboratories is to offer products and services to the medical device industry that aid in functional evaluation of technologies as related to clinical outcomes.
How We Work
Level-Set
We dedicate time to discussing the nuances of your medical device and its application, design, and technical requirements.
Brainstorm
Our technical experts ideate on the most effective and efficient way to test or simulate your device.
Sync
We present our plan to ensure we’re aligned before moving forward.
Design
The team gets to work, maintaining our rigorous quality standards and updating you along the way.
Delivery
We share our validated final test results, system, or simulation, and make sure you have what you need to keep moving forward.
Leadership

Stefan Stemmer
President & CEO
1958-2025
It is with deep sadness that BDC Laboratories announces the unexpected passing of Stefan Stemmer on April 26, 2025. Stefan led BDC with a clear vision, compassion and understanding of the needs of the company and its employees. Our thoughts are with his family and loved ones.
One of his many gifts was his ability to separate his work and home life to do the things that brought him joy…hiking and biking. Through hundreds of miles, across continents, in every season, there was a mountain to go up. When the terrain became challenging, when the miles seemed endless, he’d pause, look out at the horizon, and say with quiet hope, “Da hinten werts heller” – the encouraging alpine words that things will get better.

Craig Weinberg, Ph.D.
Chief Technology Officer/Interim CEO
Craig joined BDC Laboratories as CEO in 2006 and expanded the company from a small consultancy to a worldwide leader in testing services, test equipment and silicone anatomical models. He continues to lead BDC’s technology and innovation in his current role as CTO. Craig has 18 patents in medical device technology and test systems. He holds a Ph.D. in Mechanical Engineering from University of Colorado, Boulder, and is an active participant in ISO and ASTM working groups.

Brian Choules, Ph.D.
Vice President of Technology

Ben McCloskey
Director of R&D

Lucas Calloway
Director of Quality Assurance

Bill Carlson
Director of Sales and Marketing

Dustin Homan
Product Evaluations Program Manager

Renee Hiser
Corporate Controller

Erin Walters
Human Resources Specialist

Nate Elsen
Senior Operations Manager
Key Stats
70%
of our business is repeat customers
9in10
of our projects are custom or adapted to meet client device specifications
25+
countries are represented in our project portfolio
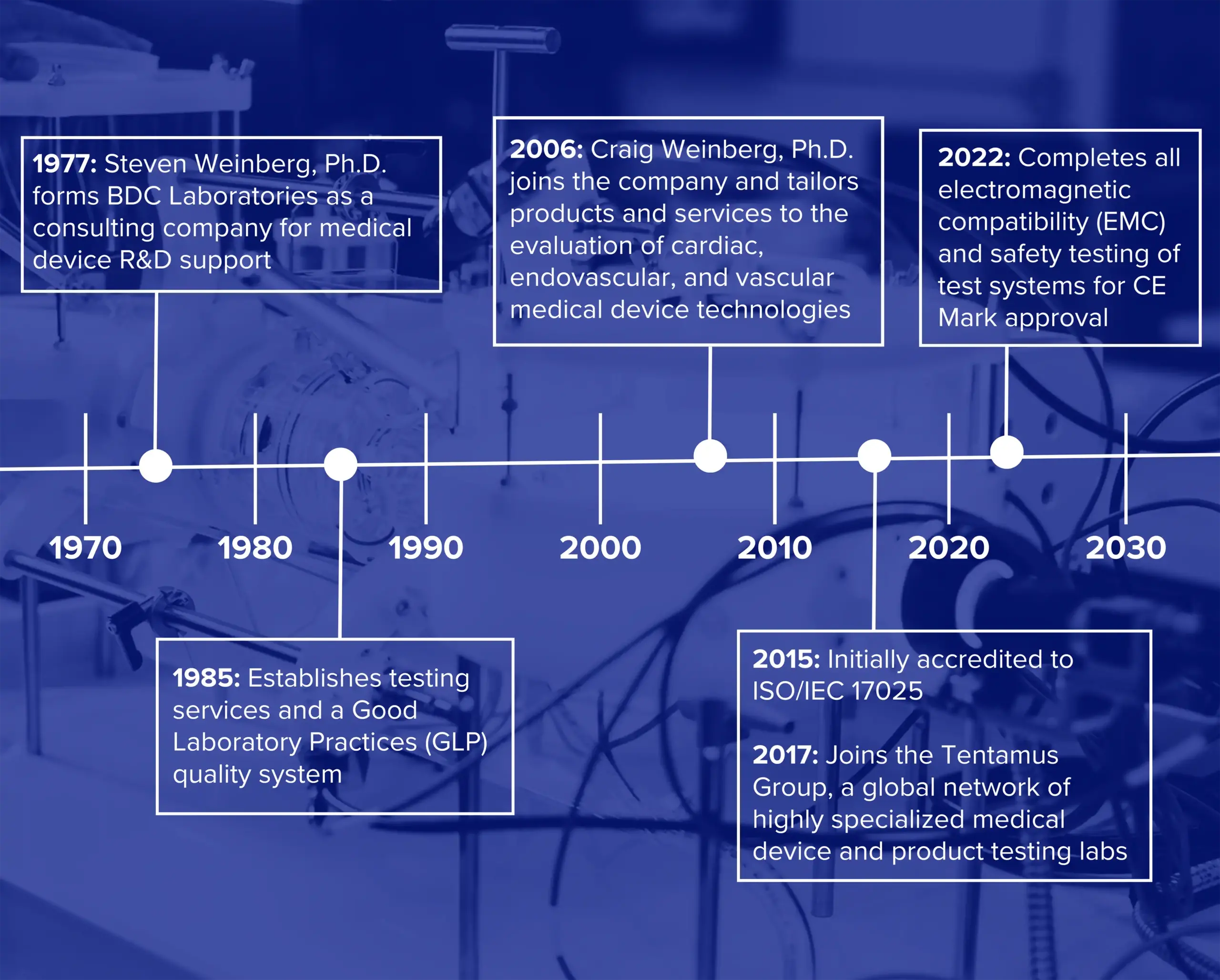
History