Testing Services
Trusted for Regulatory Submission
BDC Laboratories’ scope of accreditation to ISO/IEC 17025:2017 includes functional testing of endovascular devices, cardiac valve prostheses, vascular prostheses, and general medical devices. With more than 40 years of experience in medical device research and testing, our team combines engineering expertise with deep regulatory insight. Active participation in AAMI, ISO, and ASTM standards committees gives BDC Laboratories a unique perspective on testing for regulatory submission.
Explore a Selection of Device Technologies

Heart Valve & Valve Repair Device Testing
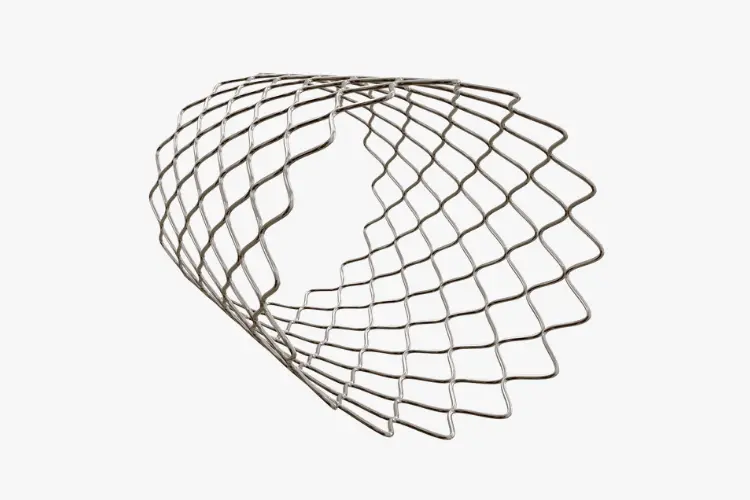
Stent & Stent Graft Testing

Catheter Testing

Guidewire & Dilator Testing

Cardiac Occluder Testing

Vascular Graft Testing

Vena Cava Filter Testing
Explore a Selection of Universal Tests
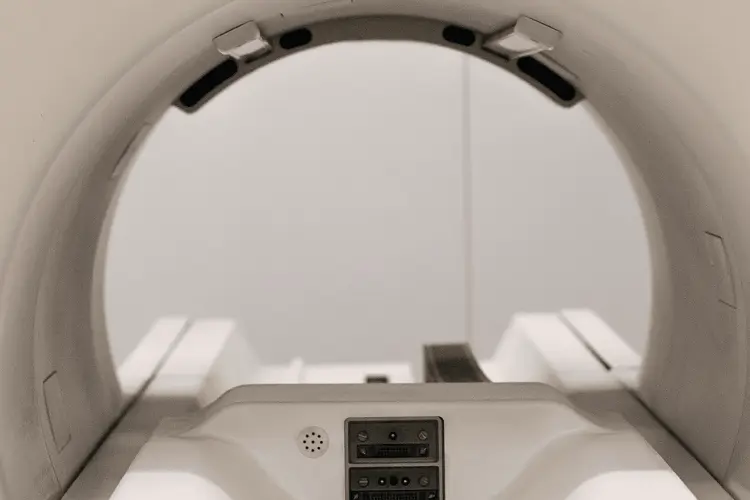
MRI Compatibility Testing

Coating Integrity & Acute Particulate Testing
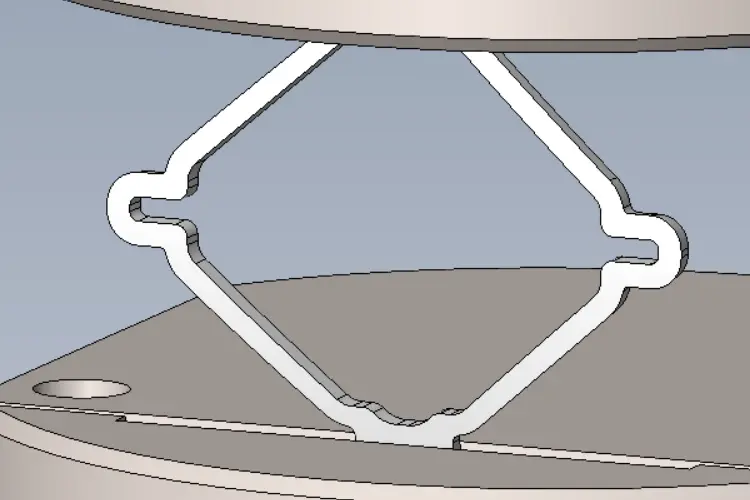
Material Fatigue Strength Testing

Medical Device Corrosion Testing
Testing Backed by ISO/IEC 17025 Accreditation
At BDC Laboratories, quality is central to everything we do. We maintain ISO/IEC 17025:2017 accreditation and comply with relevant portions of 21 CFR Part 58 (Good Laboratory Practices), among other U.S. and international governing standards. With a comprehensive quality management system, we ensure service and product integrity with a focus on regulatory compliance and continuous improvement.
Why BDC?
Built Around Your Goals
From first conversation to final report, we collaborate with your team to design and execute accredited testing programs aligned with your device goals and regulatory requirements.
Regulatory-Ready, On Time
With ISO/IEC 17025:2017 accreditation and 40+ years of industry experience, we deliver precise, regulatory-ready results on timelines that keep your project moving.
Reliable, Responsive Partnership
You’ll work with project managers who stay engaged throughout the process. They’re available for questions, quick to respond, and focused on keeping your program on track.
Explore More

Testing Equipment Brochure

Testing Services Brochure
Silicone Vessels Brochure

Simulation Solutions Brochure
Connect with an Expert at BDC
Provide a few details about your project, and a BDC Laboratories team member will respond within 2 business days to advise on next steps.

