LAA/Septal Crossing
Complete Simulated Use Solutions
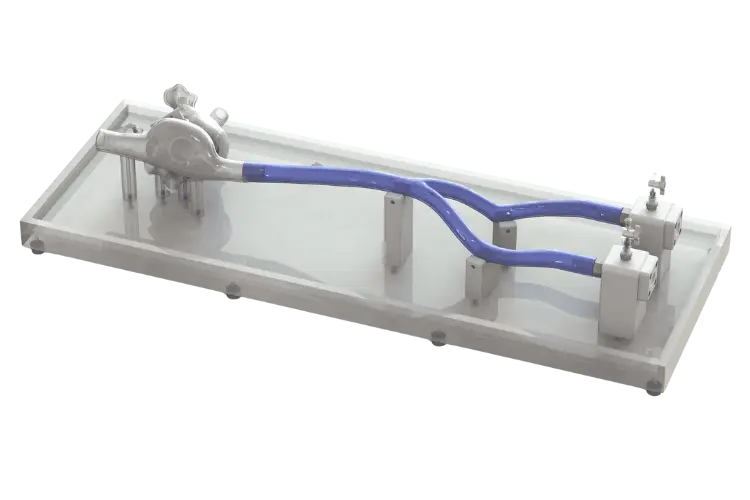
BDC Laboratories designs customizable simulated use solutions to evaluate both left atrial appendage (LAA) devices and technologies designed for septal crossing from the right to left atria.
This system features the right atrium, left atrium with replaceable atrial appendage, replaceable and puncturable atria septum, inferior vena cava, and the femoral veins. The model facilitates transcatheter delivery system access at the femoral veins, and our team can incorporate custom access routes as needed.
Key System Features
Clinically Relevant
Bench model designed for development activities, education, and physician training.
High-Visibility
Clear heart model allows better visibility of the delivery system and device.
Stable Form
Silicone vasculature is designed to ensure that the model retains its 3D configuration during procedures.
Realistic Dynamics
A replaceable left atrial appendage enables evaluation of devices across varied anatomies, while a replaceable and puncturable septal wall provides a repeatable, in situ experience for septal crossing procedures.
Anatomical Additions
Optional technologies are available to increase model fidelity and tailor systems to specific clinical use cases. These technologies support more realistic simulation and device interaction.
SLIC™ Friction Reduction Coating
BDC Laboratories’ proprietary SLIC™ Friction Reduction Coating is engineered to provide a clinically relevant surface friction profile for silicone anatomical models.
Echogenic Treatment
BDC Laboratories’ echogenic treatment alters the properties of silicone vessels to more accurately replicate how real anatomical structures appear under clinical ultrasound imaging.
Calcification
BDC Laboratories can integrate custom calcified lesions into any location with vascular or anatomical models, including coronary arteries and aortic valves.
Extend Your System
Add-ons extend your system’s capabilities. They can be purchased to support specific applications and configurations.
Fluid Handling
BDC Laboratories’ fluid handling systems deliver precise, repeatable flow conditions to support the evaluation, testing, and demonstration of cardiovascular devices and their delivery systems. Designed to simulate physiological hemodynamics, these systems range from the digitally controlled PD-1100 to the portable PD-0500 and PD-0750 platforms.
Accurate Pressure Monitoring
The Statys® Pressure Monitoring System delivers precise, reliable pressure measurements for cardiovascular device evaluation or demonstration. Supporting up to four pressure transducers simultaneously, this system combines dedicated data acquisition hardware with user-friendly software to monitor, chart, and record critical pressure signals with accuracy and consistency.
Multi-Camera Imaging
BDC Laboratories’ imaging system provides an easy, reliable way to capture high-quality visuals for flow model simulations. Configurable with up to four compact USB-compatible cameras, the system includes a tablet-based tech package with our Statys® Video Monitoring software to easily record and review multiple views of device placement in real time.
Why BDC?
Custom-Designed for Your Application
From first conversation through model delivery, we work closely with your team to create silicone vessels engineered to match your specific anatomy, clinical conditions, and testing or training goals.
Expertise You Can Rely On
With 15+ years in silicone vessel fabrication and 40+ in cardiovascular device evaluation, we design for durability and regulatory alignment as needed.
Realism That Engages and Educates
Our vessels can replicate the feel and performance of clinical use, with precise anatomy that make them ideal for hands-on physician training and sales demonstrations.
Explore More

Testing Services

Testing Equipment
Silicone Solutions
Simulation Solutions
Connect with an Expert at BDC
Provide a few details about your project, and a BDC Laboratories team member will respond within 2 business days to advise on next steps.


